The Search for an Effective Covid-19 Drug
author: Kevin Curran PhD
updated: June 29, 2020
Covid-19 is currently working its way through our global population.
This is a tragic event to watch unfold, however, one of the brightest lights on this bleak horizon is the possibility of a safe and effective pharmaceutical intervention.
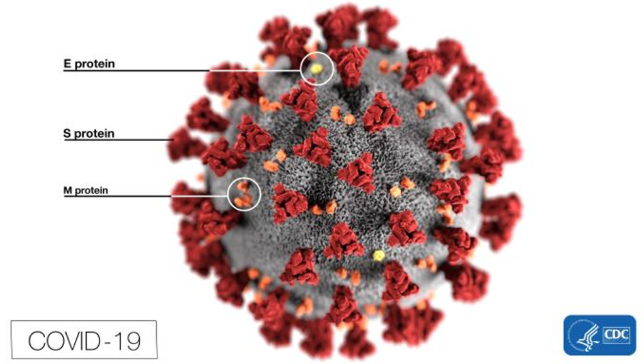
The biomedical community is hard at work designing and testing different types of drugs: antivirals, antibodies, vaccines and immunomodulators. This article serves as a resource to educate a broad audience about the latest developments in Covid-19 drug development.
Large trials have already demonstrated that a few of these drugs (i.e. dexamethasone, remdesivir) are effective treatments. In time, we will continue to discover more treatments and hopefully an effective vaccine.
On this page, I briefly describe each class of drug then discuss potential therapies in each category.
I’ll update the page each week as new data emerges from clinical trials.
Share article ↓
Click on a drug category below to learn more about the science behind each therapeutic approach.
A brief summary of expected time-frames for a Covid-19 drug is included below these drug categories.
-
Antivirals
- small molecule drugs that disrupt critical steps in the life cycle of the virus
-
Vaccines
- fragments of coronavirus are intentionally injected into healthy people to stimulate their immune system to create antibody-based immunity
-
Antibodies
- large molecule drugs attach to surface proteins on the outer envelope of virus and prevent the virus from entering our cells
-
Immunomodulators
- drugs that don’t attack coronavirus directly but instead limit the negative effects of our own hyper-activated immune system
Forecast for the time-frame of a new Covid-19 drug
Developing a new drug normally requires about 10 years of safety and efficacy testing. On top of that, the probability that an infectious disease drug will successfully advance through clinical testing and be awarded FDA approval is only 20%. The vast majority of experimental drugs fall short of a safe and effective treatment. Science is hard and clinical failures are common.
That said, during a global pandemic, the testing process can be expedited to the point where a new Covid-19 drug could possibly be approved for use in 1-2 years. But, the 80% failure rate still applies on new experimental drugs.
What are our other options?
Fortunately, we already have pre-approved drugs that may also be useful in treating Covid-19 patients. These drugs have been deemed generally safe and effective for other related diseases (e.g. HIV, malaria, Ebola, chronic inflammation). Researchers now just need to design a brief, Phase III trial to demonstrate the drug also delivers a benefit for Covid-19 patients.
- An existing antiviral, called remdesivir, has already delivered positive trial results. A five day treatment can reduce the length of hospital stay from 15 to 11 days. This drug is currently being used in many hospitals. I just wrote this article on the price for remdesivir.
- A cheap, widely available steroid called dexamethasone improves survival rates in moderate to severe Covid-19 patients. This steroid has been used since 1958 as an anti-inflammatory. Hospitals are already treating Covid patients with dexamethasone.
- Existing immunomodulators (e.g. actemra, kevara, jakavi) are already in use and could be more broadly distributed by summer 2020. These drugs suppress the harmful effects of a hyper-activated immune system. Clinical tests are underway for Covid-19.
- Plasma therapy involves the transfer of blood from a recovered Covid-19 patient to a newly sick patient. The plasma portion of recovered patient’s blood is enriched with antibody proteins that attack coronavirus. A few months of plasma therapy research could determine the best methods to deploy this strategy.
What about vaccines?
For life to return to normal on planet earth, we will most likely require a potent vaccine. The drugs mentioned above offer the chance to treat Covid-19 and minimize symptoms. However, a successful vaccine can create immunity against the virus. Once vaccinated, our body would be protected against Covid-19. A potent vaccine primes our immune system to destroy the virus whenever it enters our body.
- Multiple vaccines are in development (e.g. Moderna, Merck, J&J, Glaxo/Sanofi, CanSinoBiologics). However, before we deploy a vaccine, we need time to determine that it is non-toxic and also creates long term immunity. The Moderna vaccine is set to begin its final, Phase 3 trial in July. 30,000 healthy people will be tested. If all goes well, we could see a vaccine approved near the end of 2020.
- On June 29, an experimental vaccine produced by CanSino Biologics was approved for military use in China.

Antiviral drugs
Antivirals are small drugs that disrupt some specific step of the virus life cycle.
The list below highlights critical life cycle steps for a virus.
- Attachment to a host cell.
- Release of viral genetics into the host cell.
- Replication of viral components using the host cell’s machinery.
- Assembly of viral components into new viruses.
- New viruses release from host cell to infect new host cells.
Image below illustrates the novel Coronavirus (SARS-CoV-2) life cycle in more detail.
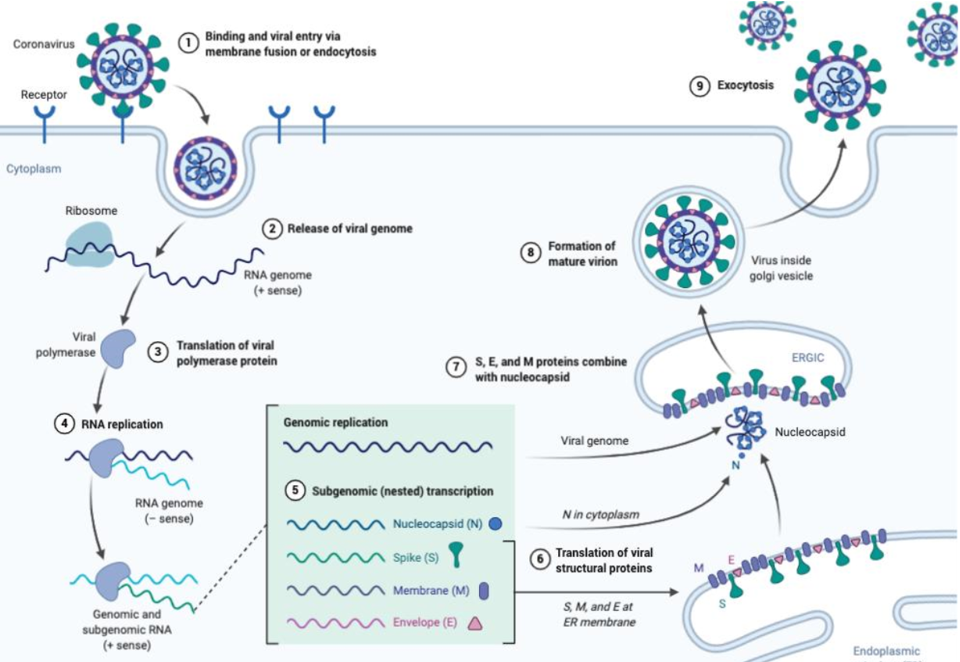
image credit: biorender.com
In order to replicate in our body, the coronavirus must first bind to an ACE-2 receptor protein on the surface of our respiratory tract cells.
Once bound, the virus enters our cell then relies on it’s RNA genome to deliver genetic code into our cells. Viral RNA translates into viral proteins, which then reassemble into capsids using our own cell’s endoplasmic reticulum as a virus construction platform. Thousands of copies, or replicants, are mass produced and exported out of our cells using our own cell’s golgi apparatus as a release mechanism.
A successful antiviral drug needs to disrupt one these critical steps in the virus life cycle.
Benefit of applying a pre-existing antiviral to Covid-19
Developing a new drug and securing FDA approval often takes 10 years. FDA approval is necessary to market and broadly distribute a drug in the US.
However, the quicker route to broadly distribute a Covid-19 drug would be to re-purpose a drug that has already received FDA approval. Fortunately, the pharma industry has already developed antiviral drugs for malaria, HIV, Ebola, influenza and other viral diseases.
In late March 2020, the World Health Organization launched a global, mega-trial to test the efficacy of the four most promising Covid-19 treatments. The trial is named Solidarity and will offer thousands of patients an antiviral treatment, either: remdesivir, chloroquine and hydroxychloroquine, lopinavir and ritonavir, or a combination of lopinavir and ritonavir plus interferon-beta.
Below, I review these promising antiviral drugs.
Remdesivir
Remdesivir is owned by Gilead Sciences, a large biotechnology company with a history of developing successful antivirals. Remdesivir is an adenosine nucleotide analog that interferes with the action of viral RNA polymerase causing a decrease in viral RNA production. Viral RNA polymerase is a common target for antivirals. Remdesivir was previously tested as a treatment for Ebola but had limited success. Approximately 500 people were treated with the drug during the Ebola crisis and remdesivir was found to be generally safe for patients and healthy people.
As of mid-June, remdesivir is the only drug that has been rigorously tested and shown to be helpful for treating Covid-19. That said, the proven benefits of remdesivir are modest: reducing hospital stays for patients from 15 to 11 days. A slight reduction in mortality was observed, but this reduction was not significant.
The FDA has not approved the drug but it has been granted Emergency Use Authorization. What does that mean?
The emergency use authorization allows for remdesivir to be distributed in the U.S. and administered intravenously by health care providers, as appropriate, to treat suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. Severe disease is defined as patients with low blood oxygen levels or needing oxygen therapy or more intensive breathing support such as a mechanical ventilator. (reference)
When the FDA does officially approve remdesivir, the world will be watching to see how this drug is priced.
Below is my thought process regarding how Gilead may price this drug (6/12/20)
Drug pricing is a bit complex. In my opinion, a company should be allowed to make a profit on a drug, but at the same time they should set a price that provides maximum access to the drug. To approach the question of reasonable drug price, we can first identify an upper and lower bounds for a reasonable price for remdesivir. The lower end of the price range would be about $10. A report determined that each 10-day treatment of remdesivir would require at least a $10 manufacturing cost for Gilead. $10 is the cost for Gilead to break even on their cost of production. However, drug companies are not looking to simply break even on their cost of goods. They want to make a profit. $4,460.00 is the upper bound of reasonable price. This number is arrived at from a value-based analysis, based on the QALY metric. Value-based pricing attempts to price a drug based on the amount of health benefit delivered to the patient. ICER, a drug pricing watchdog group, arrived at $4460 using a $50,000/QALY metric. Click here for full ICER report.
On 6/29, Gilead released their pricing structure for remdesivir. I think they struck the right balance with this pricing structure. They will make some money but more importantly, this pricing shouldn’t create too many hurdles for access.
For all governments in the developed world, including the U.S. government’s Medicaid program and the Department of Veterans Affairs, Gilead will charge $2,340 for a five-day course. U.S. insurers will pay 33% more, or $3,120. Countries in the developing world will get the drug at greatly reduced prices through generic manufacturers to which Gilead has licensed production.
It remains to be seen how significant the generic discount will be in developing nations. But the fact that, right out of the gate, Gilead has established this generic/developing world component is a step in the right direction. Click here to read my full article on remdesivir pricing and why I think Gilead settled on a fair price.
More remdesivir clinical trials are still underway.
The National Institute of Allergy and Infectious Disease (NIAID) is further testing remdesivir in a Phase 3 clinical study in adults with COVID-19.
Additionally, Gilead is conducting a trial for moderate Covid-19 patients and another trial for severe Covid-19 patients.
I’ll update when more remdesivir trial data is released.
Hydroxychloroquine (HCQ)
Chloroquine is an anti-malaria pill that’s been around since 1949. The FDA approved the drug after World War II.
HCQ has been mired in controversy ever since Covid-19 went global. President Trump has famously advocated on it’s behalf. Depending where you fall along political lines, you may hold this drug in high or low regard. Despite all the headlines, as of mid-June, clinical trials have failed to demonstrate the effectiveness of this drug as a treatment for Covid-19.
On March, 28, the FDA authorized limited, emergency use of the drug for Covid-19 patients. Since then, HCQ has been deployed as either a stand alone drug or else in conjunction with zinc or the zithromax antibiotic.
On June 15, the FDA revoked their emergency use status from HCQ.
“The FDA said that new evidence from clinical trials meant that it was no longer reasonable to believe that the drug would produce an antiviral effect.”
The FDA revoked emergency usage of HCQ after assessing data from a 11,000 person trial in the United Kingdom (Recovery). This large scale clinical trial did not find any benefit from using HCQ.
How might HCQ work?
Scientists think this chemical blocks a virus in a few different ways: 1.) by raising pH inside endosomes to make them less hospitable to a virus, 2.) by interfering with the glycosylation of ACE-2 surface receptors that bind the virus and 3.) by modulating inflammatory signals called cytokines, which are thought to be a main cause of Covid-19 death.
Since HCQ can reduce elevated cytokine activity, the drug is currently prescribed for rheumatoid arthritis and lupus (inflammatory disorders.)
HCQ is off patent and is produced under many different brand names including: Plaquenil, Hydroquin, Axemal (in India), Dolquine, Quensyl, Quinoric.
Some initial studies on HCQ looked promising, however, larger trials reveal HCQ offers no benefit for patients.
The good: A Chinese study from Wuhan showed positive benefit on a 62 person controlled trial. Another French study, led by Didier Raoult reported impressive reductions in viral load in a 36 patient, single arm study.
The bad: Another French study on 11 patients, published March 30, reported no benefit from HCQ and zithromax (an antibiotic). Additionally, doctors treating Covid-19 patients at NYU have reported safety issues with 84 of their patients using HCQ (lengthened QT intervals indicate heart issues.) Dr. Michael Ackerman, a prominent cardiologist at Mayo Clinic, stated HCQ, “could pose serious and potentially lethal risks to a small number (10%) of those susceptible to heart conditions.”
The ugly: A French trial has determined there is no benefit with HCQ. This study was performed on 181 patients hospitalized with pneumonia due to COVID-19 and who needed oxygen. Half of the patients received HCQ and the other half received placebo.
They found no meaningful difference between the groups for either transfer to intensive care, death within seven days or developing acute respiratory distress syndrome within 10 days. (reference)
On June 6, a 11,000 person clinical trial in the United Kingdom (Recovery) did not find any benefit from using HCQ.
There was no significant difference in 28-day mortality with HCQ versus usual care — and in fact trended toward increased risk of death with the active drug (25.7% for HCQ vs 23.5% for usual care.
Until we see convincing data from large, randomized, placebo-controlled trials, we cannot assume HCQ is safe and effective for Covid-19 treatment.
More large trials for HCQ are already underway.
By my last count, there are at least 10 large trials (1,500-15,000 participants) currently in progress across Spain, France, Canada and the US. One large clinical trial, sponsored by the University of Minnesota, is currently enrolling 3,000 patients and may be completed as soon as April 21, 2020. Other trials are set to complete between June and December 2020, however, early data could be released soon.
I’ll update when more clinical trial data is released.
Favipiravir
Favipiravir is an antiviral drug marketed under the brand name Avigan by a Japanese company named Fujifilm Toyama.
Avigan was previously approved in Japan and China to be used for the common flu (recurring influenza).
Similar to remdesivir, favipiravir targets the viral RNA polymerase, making it a potential treatment for Covid-19. The wishful thinking here is that the SARS-CoV-2 RNA polymerase is similar in structure and function to the viral polymerase of influenza A.
Reason for optimism: In February 2020, China’s Science and Technology Ministry official Zhang Xinmin said that favipiravir helped patients recover in an 80-day participant trial conducted in Shenzhen city. When compared to Kaletra, favipiravir treatment shortened the recovery time from 11 days to 4 days in mild and moderate Covid-19 patients. It should be noted, the favipiravir treatment also included an additional agent, interferon-alpha. We need to see how favipiravir performs on its own.
The government of Japan currently holds a stockpile of the drug.
China has already approved this drug to treat Covid-19 patients.
Early data from a Russian trial was released in June. These results show faster elimination of the virus in patients treated with favipiravir.
On average, complete elimination of the virus from the body as a result of favipiravir treatment occurred in four days, while in the standard therapy group this process took nine days.
I’ll update when more clinical trial data is released.
Vaccines
First of all, what is immunity?
Our immune system has naturally evolved the ability to produce long term immunity against many of the viruses that cross our path on planet earth.
The first time we experience a new viral infection, the B cells and T cells in our immune system will activate and attack the virus to clear it from our body. Our B cells produce antibody proteins that specifically attach to and destroy the virus. Our T cells also recognize the virus and either kill it directly or else instruct other cells in our immune system to help destroy the virus.
Throughout this process, about 10% of these activated T and B cells move into long term storage, often into our lymph nodes. We call these cells our memory B and T cells. They maintain all the necessary cellular machinery required to immediately destroy the same virus if it enters our body for a second time.
Upon re-exposure, our memory B and T cells quickly go to work and clear the virus, often before symptoms arise.
This is the natural process of acquiring immunity to a virus.
Vaccines are synthetic drugs that mimic the first round of exposure to the virus.
A successful vaccine could be:
- a weakened or dead version of the whole coronavirus
- a protein subunit from the virus (i.e. spike protein)
- a section of DNA or RNA that encodes for a coronavirus protein
- a spike protein attached to a non-replicating adenovirus
A successful coronavirus vaccine will stimulate the production of our memory B and T cells without producing toxic side effects.
At least 12-18 months of clinical trials are required to determine if a new vaccine can stimulate our long term immunity in a safe and effective manner.

image: Bruce Blaus / CC BY-SA
Reason for optimism with vaccines
Here are two reasons why viral experts are optimistic about the prospects of developing a functional vaccine against coronavirus (SARS-CoV-2).
- This coronavirus seems to mutate relatively slowly, thereby providing a stable target for a vaccine. The mutation rate for the antigen expression profile is ~22-24 mutations per year, which is relatively slow. Also, the single strand RNA of SARS-CoV-2 should favor antigenic drift over antigenic shift.
- Coronavirus infects our lungs, which is an area of our body known to receive high levels of antibodies and immune response.
That said, even with all hands on deck, Anthony Fauci, director of the NIAID, predicts getting a vaccine to the public “is going to take a year, a year and a half, at least.” Managing vaccine side effects, dosing issues and manufacturing challenges can all cause time delays.
Covid-19 vaccines in development
How do you create a vaccine?
The entire virus can be weakened or inactivated with UV light, detergents, heat or chemicals and then injected as a whole, but innocuous vaccine. Other strategies inject only the DNA, RNA, or proteins from the coronavirus with the intent of activating our immune system’s memory.
The table below lists different vaccine strategies as well as the number of organizations developing each strategy. These numbers will change rapidly.
| Type of vaccine | # of groups in pursuit | Examples of companies in pursuit |
|---|---|---|
| live, attenuated virus | 3 | Codagenix/Serum Institute of India, DZIF |
| inactivated, whole virus | 9 | SinoVac, Osaka University, Sinopharm |
| sub-units (proteins) | 52 | Novavax, Sanofi, AJ Vaccines, Baylor |
| DNA | 12 | Inovio, Zydus Cadila |
| RNA | 21 | Moderna, BioNTech/Pfizer, CureVac |
| viral vectors delivering antigens | 19 | AltImmune, CanSino Biologics, Jenner |
Below, I describe a few of the lead vaccine candidates in development.
mRNA-1273 is an example of a mRNA vaccine
RNA vaccines are a new and unproven technology. I’ve chosen to highlight the mRNA approach because, if it does work, the manufacturing and distribution process with a mRNA vaccine should be considerably faster than a more traditional vaccine approach. A successful mRNA vaccine could be the fastest route to deploy a vaccine globally.
Moderna is a clinical stage biotechnology company that is pioneering the use of RNA as a vaccine. mRNA-1273 is a mRNA vaccine against SARS-CoV-2. The RNA vaccine encodes for a stabilized form of spike protein, which is the large protein extending out from the coronavirus outer shell (envelope). Research for this vaccine came from both the McLellan Lab and NIAID’s vaccine research center.
This RNA vaccine is injected into a healthy person’s arm. The patient’s APCs, or antigen-presenting cells, receive the RNA sequence and use it to produce a version of the viral spike protein. This spike protein is then presented to our B and T cells. Our activated B and T cells then create long term immunity against any subsequent virus that contains a spike protein (aka coronavirus).
Moderna dosed their first patient for this Phase I study on March 16, 2020.
“The Phase 1 study is evaluating the safety and immunogenicity of three dose levels of mRNA-1273 (25, 100, 250 μg) administered on a two-dose vaccination schedule, given 28 days apart. A total of 45 healthy adults will be enrolled in the study. Participants will be followed through 12 months after the second vaccination.” (reference)
On May 18, Moderna released the following positive data from their Phase 1 study.
- The RNA vaccine was generally safe and well tolerated. That’s great news!
- All 45 people tested developed antibodies. People tested at three different doses all produced antibody proteins at levels that are comparable to the antibodies produced by people that were naturally infected with the coronavirus. This is a good sign. You want to see vaccine immunity that mimics the immunity seen after an infection.
- After analyzing 8 of the test subjects, a PRNA test revealed that the vaccine not only led to antibodies, but the antibodies were capable of inhibiting the virus.
It’s tricky to determine that vaccine antibodies can actually defend against the virus. Fortunately, the PRNA experiment helps measure this activity. A plaque reduction neutralization assay (PRNA) will report if an antibody can neutralize (i.e. inhibit) the deadly actions of a virus.
Moderna also revealed that their vaccine has been tested in a mouse. Mice can be vaccinated and then intentionally infected with a virus. You can’t perform that ‘challenge’ test with humans because of ethical issues. In these mice experiments, the RNA vaccine was found to prevent viral replication in the lungs of the mouse. That’s another positive sign.
The Moderna vaccine still needs to continue to prove itself in Phase 2 and Phase 3 clinical trials.
The FDA gave Moderna the green light to advance into Phase 2 studies.
The Phase 2 studies will include around 600 healthy volunteers, half of whom are 18-55 years old and half of whom are over 55 years old. They will be randomly assigned to receive either placebo or one of two doses of Moderna’s experimental vaccine. Each participant will receive two shots—early studies suggest two injections might be necessary to jump-start the immune system to generate protection against the COVID-19 virus. All the patients will then be followed for a year as the researchers monitor their immune responses. (reference)
On June 11, Moderna said their final, Phase 3 trial will begin in July. 30,000 people will be vaccinated with the 100 ug dose of the vaccine. The primary goal of the Phase 3 trial will be to show the vaccine prevents people from developing Covid-19 symptoms.
I’ll be posting updates to these trials.
CanSino Biologics
CanSino Biologics, a Chinese company, was the first group to move their vaccine into a Phase 2 study. Results from this Phase 2 study were deemed positive. On 6/29, this vaccine was approved for military use in China.
The CanSino vaccine, named AD5-nCoV, is an adenovirus vector, which means the coronavirus spike protein is attached on the surface of an adenovirus. Adenoviruses are routinely used in medical therapies. They have been genetically altered so they cannot replicate in our bodies.
Phase 1 analyzed safety data. 108 volunteers were vaccinated and received 14 days of intensive care observation.
“It is understood that everyone is in good health. Compared with the low and medium dose groups, the high-dose group had a higher proportion of high fever (body temperature ≥ 38.5 ℃), but most recovered spontaneously within 24 hours.” (reference)
The Phase 2 study analyzed a larger group of patients for a longer period of time: 125 people in the low dose group, 125 people in the middle dose group and 125 people in a placebo control group. Side effects were monitored and blood was collected throughout a six month period.
6/29/2020: Clinical trials of Ad5-nCoV vaccine in Phase 1 and Phase 2 have shown that the vaccine candidate has the potential to prevent infections caused by the novel coronavirus, however, its commercial success cannot be guaranteed, the company said. A report in the Global Times stated that data from the clinical trials of the vaccine showed a good safety profile as well as high levels of humoral and cellular immune responses.
“The Ad5-nCoV is currently limited to military use only and its use cannot be expanded to a broader vaccination range without the approval of the Logistics Support Department,” statement from CanSino.
How could we speed up the time needed to run a vaccine trial?
One prominent epidemiologist, Mark Lipsitch, suggests we try a challenge trial, but that is not an option everyone is comfortable with….
A challenge trial could shave many months off the vaccine testing period.
What is a challenge trial?
It’s a vaccine study that involves intentionally injecting healthy test subjects with coronavirus. This allows researchers to collect safety and efficacy data much quicker than the slower alternative. In the absence of intentional infection, test subjects are vaccinated then simply go about their lives for a year or so and researchers wait and see if the vaccine reduces their chances of naturally catching Covid-19. There’s an ethical cost to intentionally injecting a healthy patient with a potentially lethal virus. Some of these healthy test subjects may die. But, we have to weigh that ethical cost against the loss of life that will compile while we wait for a lengthy, vaccine trial.
Tom Frieden, a public health expert, highlights another issue with challenge trials. If we rely on young, healthy volunteers for these challenge trials, the information we learn may not be relevant to a broader population. The immune response of a 20-year old in perfect health is unlikely to model the immune response of those most at risk (i.e. older people with additional health issues).
Antibody drugs
Antibodies are proteins naturally produced by our own B cells. These Y shaped proteins specifically attach to one specific target (antigen) on the surface of an infectious agent, such as a virus. Ideally, upon coronavirus infection, our own immune system will activate and our B cells will naturally produce antibodies that will physically cover the surface of the virus, thereby neutralizing the viral activity and shutting it down. Unfortunately, this doesn’t always happen. Our immune system is not perfect.
Many pharmaceutical companies are designing antibody drugs that can be injected into our body to compliment our bodies natural response.
Using laboratory animals, scientists create customized antibody proteins that will physically attach to the surface of coronavirus. Most researchers are designing antibodies that will attach to the large Spike protein on the outside of the viral envelope.
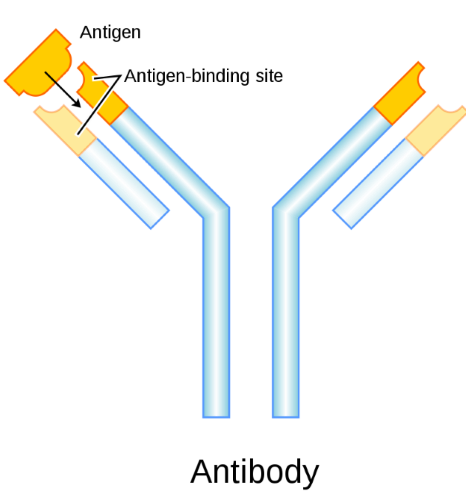
Each antibody attaches to a specific structure (antigen), similar to a lock and key.
Many companies are designing antibodies that will lock to the Spike protein on the surface of coronavirus.
An antibody drug may be infused into a Covid-19 patient’s blood once symptoms arise or the drug could be used as a preventative, to thwart a future infection.
“There’s a potential that they could be used prophylactically, which means when you look at a high-risk patient population — healthcare workers, family members of Covid-19 patients — those could be the targeted groups for a prophylactic antibody, which would essentially protect that person from being infected,” ~Andy Dunn
Organizations pursuing antibody treatments
Regeneron already has familiarity with the antibody approach to viral infection. This biopharma company previously developed a cocktail of antibodies against the Ebola virus. The Ebola drug, called REGN-EB3, is comprised of three monoclonal antibodies. In 2019 Congolese health officials reported these antibodies were effective at treating Ebola virus disease.
Regneron has been busy designing a combination of antibodies to be used against coronavirus (SARS-CoV-2). They generate virus-neutralizing antibodies from their VelocImmune mice, a genetically engineered mouse that generates human antibodies against SARS-CoV-2.
On June 11, Regeneron announced the start of the first clinical trial for their dual antibody cocktail drug for the prevention and treatment of COVID-19.
This new antibody drug is called REGN-COV2.
Vir Biotechnology, Biogen and Takeda are also developing antibody drugs against Covid-19.
It’s difficult to predict a timeline for the approval of a new antibody drug.
Plasma Therapy
When you survive a coronavirus infection, your blood becomes filled with antibody proteins that can attack the virus.
Is it possible to donate your blood and help patients currently hospitalized with Coivd-19?
The answer seems to be yes and this process is called plasma therapy.
Plasma, or serum, is the yellowish liquid component of our blood. This portion of blood is filled with antibody proteins. Once someone is fully recovered from Covid-19, they can make their way to a blood collection clinic and make a donation. After a screening process, a plasmapheresis step and an antibody check, the blood can be infused into recipients. New York City Blood Center is currently accepting plasma donations.
Scientists think this could be an effective treatment for patients hospitalized with Covid-19 or those who are at high risk of getting sick, like health care workers and people with weak immune systems.
Plasma therapy seems to be most effective when administered early.
“Antibodies are really good at preventing the infection, and they’re probably good at preventing the progression of infection when they’re given early on,” says Shmuel Shoham, MD, an associate professor of medicine at Johns Hopkins University who is part of the Covid-19 Convalescent Plasma Project. “But once the disease is deep in the tissues, we think its effectiveness is limited.” ~Emily Mullin article
Early research on Covid-19 plasma therapy suggests this approach can improve conditions. A Chinese study revealed that seven out of 10 patients who received plasma experienced sharp reductions in viral loads.
Further research is necessary but this approach could be deployed broadly in a few months.
Immunomodulators
Immunomodulators are drugs that help regulate or normalize our immune system.
When our body is under attack from infection, such as a virus, the cells in our immune system rapidly activate in attempt to defeat the infection. These immune cells secrete chemical signals, called cytokines, which travel throughout our body and instruct other immune cells to also activate and help eliminate the virus. For the most part, this is beneficial as we want a hyper-activated immune system in a moment of infection. However, it is possible to have too much of a good thing.
A hyper-activated immune system, with an excessive amount of cytokine signalling, can damage our own lung tissue and eventually kill us.
Evidence suggest this novel Coronavirus can provoke an overreaction from our immune system, resulting in a cytokine storm. Two particular cytokines, interleukin-6 (Il-6 ) and C-reactive protein (CRP) seem to be key signals in the Covid-19 related cytokine storm.
Immunomodulator drugs engage with specific cytokines in a manner that suppresses our immune activity, thereby minimizing tissue damage in our body.
Actemra
Actemra (tocilizumab) is an immune suppressing drug. It’s already FDA approved to treat rheumatoid arthritis and symptoms related to CAR-T cancer therapy.
Actemra is a large molecule, antibody drug that specifically disrupts one particular cytokine, named interleukin-6 (IL-6).
When this antibody drug attaches to IL-6 receptors it prevents the cytokine from over activating our immune system. In this manner, Actemra could reduce the cytokine storm, which contributes to lung tissue damage in Covid-19 patients.
A small, single-arm, 21-patient study in China showed Actemra reduced fevers and need for supplemental oxygen in Covid-19 patients. Actemra is already in use around the world to help regulate Covid-19 patient’s immune response. That said, we still need to see data from large, controlled clinical trials to learn about best dosage and timing for administration.
The FDA has approved a Phase 3 trial to allow parent company, Roche, to assess Actemra in Covid-19 patients. This trial is specifically recruiting for Covid-19 patients with pneumonia.
I will update once we see trial data results.
Kevzara
Another IL-6 inhibiting antibody drug named Kevzara (sarilumab), is also being explored as an option to reduce hyper-activated immune systems. Parent companies, Regeneron and Sanofi, have launched a Phase 2/3, double blind, trial to asses Kevzara’s ability to reduce symptoms in severely ill Covid-19 patients.
I will update upon seeing results from this Kevzara trial.
Jakavi covid
Pre-clinical data and independent studies suggest that inhibiting the JAK receptor kinases can also reduce the cytokine storm that leads to life-threatening complications in Covid-19 patients.
In early April, Novartis and Incyte announced plans to initiate a Phase III clinical trial to evaluate the use of Jakavi, their JAK inhibitor drug, for the treatment of severe Covid-19 symptoms.
Jakavi, an oral inhibitor of JAK1 and JAK2 tyrosine kinases, has already been FDA approved to treat a hyper-activated response seen in graft versus host disease.
“The proposed blind, double-arm study will evaluate a combination of Jakavi alongside standard-of-care therapy to treat COVID-19 patients with pneumonia.” (reference)
I’ll update once we see how well Jakavi works an an immunomodulator for Covid-19.
Thanks for reading!
I hope this page has oriented you with the landscape of Covid-19 drugs. I wrote the first draft of this article while ‘sheltered in place’ during the first week of April, 2020. My state of California has been on lock-down for over a week. Our national deaths/day number has been rising rapidly and NYC is currently taking a beating.
I hope each time I update this page the Covid-19 situation looks better than it does today.
Wash your hands!
Kevin Curran, 4/3/2020