author: Kevin Curran PhD
updated: 3-12-2020
Scientists have been experimenting with stem cells for almost 40 years.
Which treatments look promising? Are there any legal stem cell therapies in 2020?
What’s the story with all these quasi-legal, stem cell clinics popping up around the United States?
You can click on each topic below or scroll down and read the whole page.
Stem cell biology is a fascinating field of science. The field combines traditional developmental biology, cutting edge genetics and an intoxicating vision of new organs generated with personalized stem cells.
Hello friends! I just made the adjacent stem cell video to accompany this article.
Click on the movie for a brief review of the current status of stem cell therapies.
~Kevin
After college, I was immediately lured into this universe. I spent the next seven years working as a stem cell scientist at UC San Francisco and the University of Washington. During my time in the lab, I gained a healthy respect for the complexity of the subject. I still recognize the awesome potential of stem cells but I also appreciate the inherent challenges in this world…
- The genetics of cell development are intricate and convoluted.
- Successful results observed in lab animals (i.e. mice) don’t always replicate in human patients.
- Transplanted stem cells don’t always function in the correct manner. Sometimes, they develop into the wrong cells.
- Our immune system often rejects freshly transplanted stem cells.
As of 2020, cord blood therapies are the only FDA approved, stem-cell based therapies in the United States. Cord blood therapies are currently limited to treating patients with blood disorders. The FDA has approved other cell therapies (CAR-T and skin/cartilage cell injections) but that’s not the same as treating a disease with a stem cell therapy.
On an international level: Europe, Japan, India and South Korea have seen a handful of approved stem-cell based products. Most of these internationally approved products utilize a stem cell population called mesenchymal stem cells (I’ll explain below).
The FDA’s reluctance to approve more stem cell therapies is noteworthy. FDA approval is difficult and often takes 10-15 years from initial research to market approval. That said, the bio-medical community has been pursuing stem cell therapies for a similar amount of time as they’ve been pursuing CAR-T and gene therapy, yet both of those types of medicine have delivered FDA approvals.
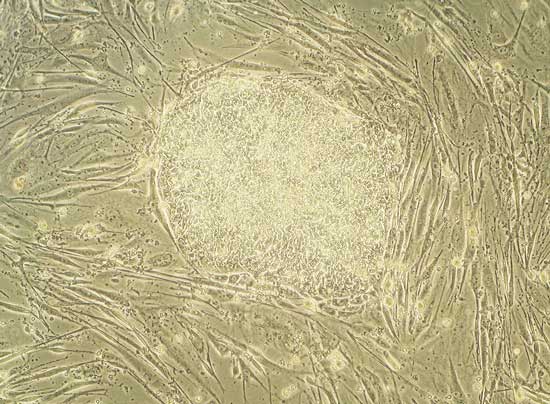
Human embryonic stem cells growing in a dish.
These cells can develop into each of the 200 different cell types in the human body.
The truth is…pharmaceutical companies are having a hard time convincing the FDA that stem cell therapies are viable. The FDA sets a high bar for efficacy and safety and many stem cell treatments have fallen short of that threshold.
In contrast, quasi-legal, stem cell clinics have been very quick to manifest all across America. At last count, about 800 stem cell clinics are offering non-FDA approved therapies in the U.S.
What exactly are these clinics selling? In late 2019, the FDA updated their warning to consumers about illegal and potentially harmful stem cell clinics. The FDA has sent warnings to certain clinics that offer the most misleading advertising. But for the most part, the FDA doesn’t have enough personnel or budget to investigate all the clinics. They encourage the consumer to do their own homework and be cautious when considering a therapy.
This stem cell page serves as a resource for people who wish to stay on top of the evolving world of stem cell biology.
I cover some basic science, discuss the latest breakthroughs in clinical therapies and explain the situation with these non-FDA approved clinics.
The page will be updated often to reflect new developments.
If I’m missing any key information, please let me know.
What are stem cells and why are they special?
Stem cells are a remarkable population of cells that exist in many tissues of the human body.
Stem cells are famous for their ability to develop into most of the different cells in our body.
These cells can recognize injury in our body and then re-populate the damaged tissue with a fresh supply of specialized cells. In this manner, stem cells work hard to keep us healthy throughout our entire life. They can act as an internal repair system. In some of our organs, such as our gut or bone marrow, stem cells regularly send out replacement cells to repair worn out tissue.
The other defining feature of stem cells is their ability to renew themselves for long periods of time. Stem cells can stay tucked away in our body. They lay low and divide and remain a discrete and healthy population of cells. During this latent state, they remain un-differentiated. Un-differentiated means they have not yet developed into specialized cells. These latent stem cells are waiting for a signal from our body. Once they receive the correct signal, these stem cells will divide and form the specialized cell type that is needed. This could be a brain cell a muscle cell a skin cell…whatever cell is needed.
The stem cell division image on the right illustrates all the key features of stem cell development. By following along with these cell divisions, you see how stem cells can either divide indefinitely and remain as undifferentiated stem cells (1)….or they can respond to signals from our body and travel down a particular developmental pathway (2,3). Most developmental pathways will eventually differentiate the stem cell into one specific cell type (4).
In summary, stem cells keep us healthy by supplying our body with fresh cells throughout the duration of our lives.
If this is understood, then it’s easy to appreciate why scientists are working so hard to use stem cells to treat human disease.
Once we learn to effectively manipulate stem cells, we can then direct their regenerative abilities at a patient’s degenerate tissue or organ.
Before we get into the latest advancements, let’s first orient ourselves with a brief history of stem cell research.
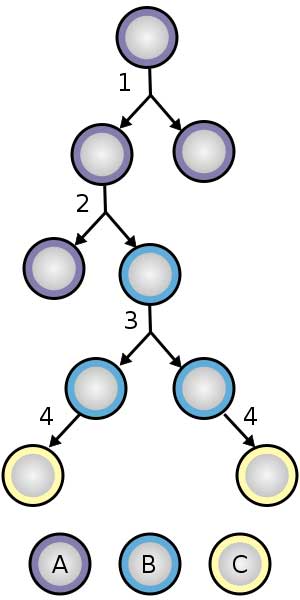
Stem cell division and differentiation.
A: stem cell
B: progenitor cell
C: differentiated cell
Deep history of stem cell therapy
Stem cells have enjoyed a colorful history – filled with politics, ethical debates, bogus claims, controversy and a lot of hype. Let’s work our way through some of the major events.
Going way back to the discovery of cell theory.
To place our knowledge of stem cells in the proper context, we should first discuss a group of very bright German physicians. In 1838, Theodor Schwann and Matthias Schleiden proposed the cell theory of life. Schleiden and Schwann were the first scientists to acknowledge that all life is built from cells.
A few years later, Rudolf Virchow built upon Schleiden and Schwann’s cell theory by proposing that cells divide to create other cells. This may seem obvious to us now, but it certainly wasn’t obvious back then.
Prior to the mid-1800’s, people thought that diseased cells could spontaneously generate in a sick person’s body. Virchow proposed that diseased cells arose from a malfunctioning version of a normal cell.
In 1855, Virchow summarized his contribution to biology with the pithy phrase, Omnis cellula e cellula.
translated – Every cell stems from other cells.
The field of stem cell biology rests on this conceptual framework.
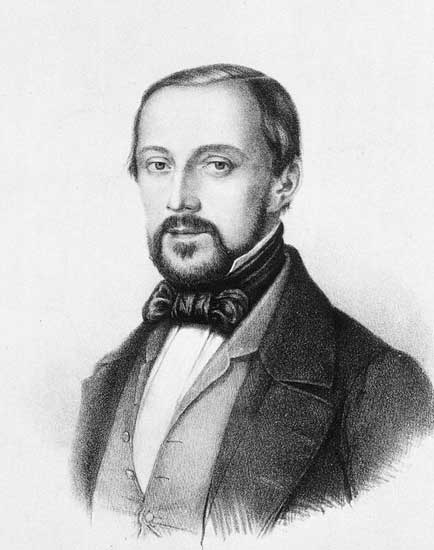
Rudolf Ludwig Carl Virchow
During the next 100 years, modern medicine continued to plow forward.
Peering into microscopes, physicians began to appreciate the different features and functions of the 200 cell types that make up the human body.
How did the first stem cell therapy emerge?
Oddly enough, the discovery of stem cells arose from an incident related to the bombing of Japan in 1945. When the atomic bombs fell on Hiroshima and Nagasaki, thousands of people perished instantly. Adding insult to injury, the Japanese survivors of these brutal bomb attacks began to die off in the years following 1945. A massive amount of atomic radiation was released when the bombs exploded. This atomic radiation did not kill the Japanese immediately but it did destroy the stem cells that lived in their bone marrow. We now know these bone marrow stem cells develop into all the cells in our blood and immune system. These stem cells are called hematopoietic stem cells.
In 1956, the science community figured out the relationship between bone marrow, stem cells and the demise of Japanese survivors. When the Japanese survivors lost their bone marrow stem cells, their bodies lost the ability to create new immune system cells. Without white blood cells, the survivors died off as soon as an infection entered their body. They simply had no immune system to challenge even a mild infection.
This knowledge led to the first bone marrow transplant in 1968.
By transplanting bone marrow from one person to another, you are bringing fresh stem cells into a sick patient’s body. These hematopoetic stem cells can then proliferate and supply the sick individual with different types of blood and immune cells. The first bone marrow transplant successfully treated two siblings suffering from SCID, or severe combined immunodeficiency.
Moving into the modern era
In 1978, a separate stem cell population was found in cord blood. Cord blood is the blood left over in the umbilical cord and placenta after childbirth. Cord blood stem cells function in a similar manner to bone marrow stem cells.
In 1981, MJ Evans and MH Kaufman, published a landmark Nature paper that demonstrated how stem cells could be isolated from mouse embryos and then grown indefinitely in a petri dish in a lab. These immortal stem cells showed normal cell behavior (normal karyotype) and retained their ability to develop into multiple cell types. This paper was significant because it meant that stem cells could be studied and manipulated in a lab environment.
At this point, the stem cell field began picking up steam. In the 1990’s, scientists were learning how to manipulate adult mouse tissues to produce different cell types. For example, scientists could isolate bone marrow cells in a flask and then tweak with the media conditions to produce nerve or liver cells. Media is what we call the nutrient rich broth that feeds stem cells as they grow in a lab. Controlling the direction of stem cell development often means adding or subtracting biological signals (proteins or small chemicals) from the cell media.
In 1998, researchers at the University of Wisconsin isolated cells from the center of human embryos. These cells are known as embryonic stem cells. They are the holy grail of stem cell populations, because they retain the ability to become all the cell types in a human body. In contrast, stem cells from bone marrow (hematopoetic stem cells) can only differentiate into the blood cells and immune system cells.
Also in 1998, the work of Janet Rossant, a developmental biologist, helped lead to the discovery of the trophoblast stem cells. The trophoblast cell lineage is critical to the survival of mammalian embryos in utero. Since the discovery of the trophoblast, scientists have been able to use these cells to research congenital abnormalities in the blood vessels and heart.
The US government restricts human embryonic stem cell research
Stem cell scientists quickly became very intrigued with these human embryonic stem cells. The cells offered scientists a chance to learn how human stem cells can either remain undifferentiated or be pushed down different developmental pathways. However, there was a problem. Human embryonic stem cells (hESC) were often collected from leftover fetuses from in vitro fertilization clinics. In vitro fertilization generate multiple embryos. Many of these will not be implanted in a women. With proper consent, the extraneous embryos can be donated to stem cell science research. This may seem innocuous enough, however the word embryo is a hot button word in the United States. The pro-life and religious right were uncomfortable with any manipulation of human embryos, even if the embryo were destined to be discarded. It didn’t help that some researchers collected their discarded embryos from abortion clinics.
Is stem cell therapy legal in the US?
In the late 90’s, stem cell research in the U.S. was picking up steam. It fell upon president George W. Bush to weigh in on the issue of human embryonic stem cells. On one hand, the government wanted to support cutting edge science but on the other hand, politicians didn’t want to endorse the harvesting of human embryos. On February 2001, George W. Bush, requested a review of the NIH guidelines. On August 2001, Bush made his decision and enacted a policy to limit funding for embryonic stem cell research to 19 pre-existing human embryonic stem cell lines. This was a huge setback to the stem cell research community.
Fortunately, state level funding is also a possibility. California took the lead in state sponsored stem cell research. In 2004, California voters approved $3 billion in funding for stem cell research. I remember this moment vividly. In 2004, I was an embryonic stem cell scientist in the Conklin Lab at UCSF. This was an exciting time. The state level funding was a breath of life into hESC research.

President George W. Bush
Throughout the 2000s, it became clear that the federal restriction on human embryonic stem cell research was misguided and constituted federal overreach. On March 9, 2009, President Barack Obama issued an executive order that removed many of these federal restrictions. That was a good moment for the stem cell community. However, the unfortunate reality is that any stem cell science involving human embryos will always be politically charged. I wouldn’t be surprised if the pendulum swings back to tighter regulations in the future.
Scientific fraud rocks the international stem cell community
We should also take a moment to reflect on an unfortunate incident that ballooned into an enormous international scandal. In 2005, a Korean veterinarian and scientist named Woo Suk Hwang published a breakthrough paper in Science. Hwang claimed that his group at Seoul National University had successfully cloned human embryos. Hwang performed this remarkable feat through somatic cell nuclear transfer (SCNT). This means you collect human eggs, pop the egg nucleus out and pop in an adult nucleus from another adult human. You then allow the egg to develop into an embryo (a new human). In theory, the new embryo is a clone of whomever donated the adult nucleus. The science community already knew cloning was possible with other animals (sheep, mice, cows, ext.) Yet, nobody had succeeded in cloning a human. Hwang claimed he had accomplished human cloning and he also claimed he derived embryonic stem cells from this cloned embryo. The international community was very impressed…for about a year.
In 2006, errors and inconsistencies began to surface in Hwang’s scientific documentation. Hwang’s colleagues at Seoul National University intiated a full investigation of all his published claims. Upon closer scrutiny, everything fell apart. On January 10, 2006, the University issued a statement proclaiming that all of Hwang’s data was fabricated.
Deriving stem cells from a cloned human embryo
Unfortunately, Hwang’s false claims cast a shadow on the integrity of the entire stem cell community. The somatic cell nuclear transfer technique was legitimate, it just wasn’t quite ready for prime time. Human cloning aside, there is possibly immense value in using SCNT to generate embryonic stem cells that are a perfect match for a human adult. The human that donates the adult cell nucleus to the SCNT procedure will then generate a private reserve of human embryonic stem cells, which can be stored in a freezer for decades. In theory, if a scenario arises, later in life, when that adult needs some replacement tissue, the hESC can be thawed down and differentiated into the desired cell type, then transplanted into the same adult. Since the genetics are the same, there shouldn’t be an issue with Graft-versus-host-disease (GVHD), aka immune rejection of another person’s transplanted cells.
It took scientists another 5-7 years to work out the human SCNT details. In 2013, a team of scientists led by Shoukhrat Mitalipov published the first legitimate report of human cloning. Mitalipov also pulled embryonic stem cells from the cloned human embryos. His results have now been replicated by numerous other laboratories. So, it is safe to say, we have figured out how to clone human embryos. However, it’s still too soon to regard this procedure as a tool for an adult to generate their own reserve of hESC (with therapeutic value). Mostly because scientists are still sorting out the details on how to best perform therapies with hESC.
It should also be noted that in all of these experiments, the cloned human embryos were never implanted into a women. In most nations, it’s illegal to implant a cloned human embryo into a women and then bring that cloned baby to term. If this final step is completed successfully, we would then have human clones walking the earth. As far as I know, there is no organization or government that is pursuing this objective. Of course, no one can be absolutely certain what happens in discreet, privately funded labs. If anyone has any information about private labs that are cloning human embryos and attempting to bring them to term, please contact me here.
Current Stem Cell Research
Stem cell research is moving forward in many different directions. The human body is a source of multiple types of stem cells. Many of these cell types are being explored for their therapeutic potential (neural stem cells, mesenchymal stem cells, adipose stem cells, human embryonic stem cells, etc.)
Below, I will touch on some of the most promising advances in this stem cell universe. I’ve organized the categories of stem cell research by either the tissue/organ that scientists are trying to regenerate or by the type of stem cell that is being pursued for therapeutic value.
2020 update:
It brings me no great pleasure to report that little progress has been made in our attempts to convert stem cell research into approved medicine. Results from clinical trials using stem cells to address Parkinson’s, Alzheimer’s, heart issues, stroke, paralysis, etc. have failed to convince the FDA they are worthy of drug approval status. Incredibly brilliant and hard working scientists continue to plow forward, however, in the absence of approved medicine…funding will eventually dry up. This story is playing out in the state of California. Stem cell scientists benefited from a $3 billion dollar program created in 2004. This program is called California Institute for Regenerative Medicine (CIRM). As of late 2019, CIRM’s money was beginning to dry up.
CIRM supported infrastructure, basic research, and training early on, in the past 3 years it has poured most of its remaining $759 million into clinical trials—a total of 55 of which are ongoing or completed to date—as the agency faced pressure to produce the medical treatments its supporters were initially promised. (Science)
CIRM is now hoping Californians will once again vote to approve more taxpayer money for stem cell science. A funding initiative will be voted on in November 2020.
That said, there is plenty of room for optimism. I am incredibly impressed with clinical trial results in the multiple sclerosis space. It seems mesenchymal stem cells do seem to create very real improvements in patients. I’ll cover this in more detail below.
Clarifications and definitions
It should be noted that the political debate regarding stem cells (described above) only pertains to human embryonic stem cells. Most of the clinical trials and treatments described below involve adult stem cells, not embryonic stem cells. Embryonic stem cells are removed from a human embryo when that embryo is just a few days old. In contrast, adult stem cells exist in many parts of an adult human body. A clinician can extract these cells fairly easily without hurting the individual. There is no ethical issue concerning the use of adult stem cells.
Ok, first let’s review some therapy terminology. Stem cell treatments are classified as either: autologous, allogenic or xenogenic.
Autologous: stem cells are obtained from an individual, processed in some way, then transplanted back into the same individual.
Allogenic: stem cells are obtained from an individual, processed in some way, then transplanted into a different individual from the same species. For example, bone marrow can be removed from one person and transplanted into another person.
Xenogenic: stem cells are removed from one species, processed in some way, then transplanted into an individual from another species. For example, stem cells can be removed from a cow and then used to treat a human.
Heart tissue regeneration
One cluster of scientists are focused on using stem cells to repair damaged heart tissue. After a heart attack (or myocardial infarction) a section of the heart is often scarred. This means that a region of heart cells (cardiac-myocytes) are no longer functional and they no longer beat in synchronicity with the heart’s natural pacemaker. In essence, the scar region becomes dead weight for the pumping heart. Stem cell transplants performed in laboratory animals suggest that damaged heart tissue could be repaired with stem cells. On February 2012, researchers at Cedar-Sinai Medical Center and John Hopkins published clinical trial results showing this strategy could also work in humans. Adult stem cells were extracted from a patient’s heart after the patient experienced a heart attack. These stem cells were grown and treated in the laboratory, then transplanted back to the damaged area on the patient’s heart. This treatment decreased scarring, led to regrowth of heart tissue and returned some functionality to the damaged organ. These results were regarded as the first demonstration of stem-cell based, therapeutic regeneration in humans.
Unfortunately, follow up trials have failed to demonstrate that stem cells can safely and effectively improve heart function.
Hundreds of millions of dollars have been spent on cardiac stem cell research over nearly two decades now without any sign of success or progress. (MedPage)
As of 2020, there are no FDA approved stem cell treatments for heart regeneration.
Eye tissue regeneration
On February 15, 2015, results from a FDA approved clinical trial demonstrated the ability of stem cells to reduce blindness.
Macular degeneration is one of the leading causes of blindness in developed nations. This eye disease features the degeneration of critical eye tissue called retinal pigment epithelium (RPE). Once your RPE begins to fall apart, you can lose your photo-receptor cells and then, ultimately, your vision will suffer. Eye tissue is an ideal part of the body to perform stem cell transplants for two reasons. 1.) The eye is an easy part of the body to access. 2.) Eye tissue is immuno-privileged, meaning that eye tissue is less likely to reject transplanted cells.
A stem cell transplantation will often illicit a strong immune system response, which needs to be addressed with immuno-suppresive treatment in order to avoid immune rejection. But, because the sub-retinal space is sanctioned off by the blood-ocular barrier, RPE tissue has a higher tolerance for foreign antigens or non-histocompatible cells (such as laboratory treated stem cells).
That is the fancy way of saying, eye tissue is less likely to reject transplanted stem cells.
In 2015, Steven Schwartz and colleagues treated 18 patients suffering from macular degeneration. Human embryonic stem cells were transplanted into the eyes of these blind patients. This Schwartz study, published in The Lancet, reveals that 10 of the eyes treated responded with improvements in visual acuity.
There are currently no FDA approved stem cell treatment for eye diseases.
However, in February 2015, the European Commission (EMA) approved Holocar to use in cases of blindness caused by burning.
Annually, only about 1,000 people annually in Europe will be eligible for Holocar treatment. A candidate for this treatment would include burn victims who have become blind but whose eyes have not been completely destroyed.
Graziella Pellegrini, an Italian scientist, was the main driver of this eye therapy. Graziella has been doggedly pursuing stem cell eye regeneration for 25 years. She led her team through clinical trials showing damaged corneal cells could be replaced with stem cells.
“I had seen patients who had starting seeing again after 20 years of blindness: how could I stop?”
Her group formed a spin-off company, called Holostem, to help convert these clinical results into an approved therapy. Holostem and Chiesi Farmaceutici (Parma, Italy) jointly developed the Holocar therapy as a commercial product. This autologous Holocar treatment removes healthy corneal cells from a burn victim with partially damaged eyes. The corneal cells are then cultivated on a bio-engineered support made from modified human fibrin protein. When approximately 3,000 stem cells have generated on the fibrin platform, the stem cells and their fibrin support are transplanted onto the damaged eyeballs. This transplant is sufficient to seed the growth of fresh and functional corneal eye tissue.
In 2018, Holostem was granted Orphan Drug Destination from the FDA. This could speed the drug’s movement through clinical trials. As of 2020, I have not heard any updates on clinical trial progress. Their status on clinicaltrial.gov states they are still recruiting for trial.
Skin tissue regeneration
Skin regeneration is a tricky endeavor. For quite some time, scientists and engineers have been tweaking with experimental designs, trying to properly generate new skin tissue and graft it onto a patient’s body. A Nature paper published in November 2017 marks a major milestone in this noble pursuit. A team of Italian researchers, led by Michele De Luca, came to the aid of a young man suffering from a horrible skin disease called JEB, or junctional epidermolysis bullosa. Patients suffering from JEB carry mutation in a gene called laminin-332. In a healthy individual, this gene encodes for a crucial basement-membrane protein that allows skin cells (aka keratinocytes) to function normally. If you harbor a mutation in your laminin-332 gene, then your skin cells won’t behave correctly. In this case, Italian researchers focused their efforts on a seven year old German boy with a mutated laminin-332 gene. This unfortunate child had lost 80% of his skin.
The Italian scientists removed a small portion of his remaining skin. They then isolated clusters of epidermal stem cells from his skin tissue. Once isolated in a lab, they genetically modified his epidermal stem cells. A virus was employed to re-introduce a healthy version of the laminin-332 gene. These genetically modified stem cells were then grown into sheets in a lab and – when the time was right – the sheets of enhanced skin cells were grafted onto the boy’s body.
The scientists confirmed that these genetically modified stem cells had taken over the job of repairing the boy’s skin.
The patient was discharged in February 2016. His epidermis is currently stable and robust, and does not blister, itch, or require ointment or medications.
This is truly amazing. What a gift for the young boy! Scientifically, the paper combines exceptional progress in both gene therapy and stem cell transplantation.
There are currently no FDA approved skin/stem cell based therapy products.
induced Pluripotent Stem Cells (iPSC)
I was deep into the rabbit hole of my doctoral thesis in 2006. My PhD project attempted to identify the transcription factors that could shift a stem cell into a differentiated cell. I mention this because in 2006, I distinctly remember our Monday morning lab meeting where we first discussed a breakthrough paper published by Shinya Yamanaka. Working from Kyoto University, Yamanaka and his grad student Kazutoshi Takahashi, figured out how to re-program adult cells back into pluripotent stem cells. His team took skin cells from a middle-aged volunteer and treated the cells with just 4 genes. This relatively simple genetic re-programming created a stem cell population from adult skin cells. This was a huge finding. The discovery showed it was possible for adults to create their own stem cells from their own skin cells. In theory, these personalized stem cells could be used to replace any deteriorating tissue later in life. For this achievement, Yamanaka shared the Nobel Prize in 2012.
In a 2017 NY Times interview, Yamanaka describes how iPSC are now being explored as a tool to treat macular degeneration. Dr. Takahashi, working at Riken, removed skin cells from a 70 year old macular degeneration patient. The skin cells were first re-programmed to become pluripotent stem cells. They were then encouraged to become adult retinal cells (by tweaking with signal factors). Finally, these retinal cells were transplanted into the eyes of the original macular degeneration patient. According to Yamanaka, this 70-year old woman now sees much brighter. Yamanaka also acknowledges that subsequent treatments were not as successful (problematic DNA mutations arose in the altered iPSCs).
Yamanka expects great things from iPSC in the future, but he also often reminds the public that they need to be patient while the reasonable clinical applications are explored.
2019 update: NIH researchers published a report detailing how iPSCs can work to replenish the photoreceptors that become damaged in advanced AMD (a cause of blindness). This work was performed in rat and pig models but now appears ready to move forward into human clinical trials. In this report, human blood cells were removed from patients, these cells were re-programmed into iPSC cells, then the cells were encouraged to become retinal pigment epithelium cells. These RPE cells were transplanted into the eyes of animals with advanced AMD. This procedure helped restore vision to the animals.
Ten weeks after the human iPSC-derived RPE patches were implanted in the animals’ retinas, imaging studies confirmed that the lab-made cells had integrated within the animal retina.
Researchers also confirmed that this process did not introduce genetic mutations in the DNA of these re-programmed cells.
There are currently no FDA approved iPSC stem cell therapy products.
Bone marrow stem cells
As described in the previous section, bone marrow transplants have been performed in the U.S. since the 1960s. Our bones are filled with a very fertile population of bone marrow stem cells, these can be applied for a variety of therapies.
Bone marrow is an excellent source of CD34+ stem cells, also known as our hematopoietic stem cells. This famous class of stem cells can develop into all of the red blood cells, white blood cells and platelets in our circulatory system. The bone marrow stroma also contains a separate class of stem cells called mesenchymal stem cells (MSCs). MSCs are also mutlipotent and can develop into fat cells (adipocytes), bone cells (osteoblasts) and connective tissue (fibroblasts), chondrocytes and myocytes. Unfortunately, the bone marrow seems to be a relatively poor source of mesenchymal stem cells. These bone -marrow derived MSCs may provide support for tissue regeneration via improving blood flow (revascularization) and by providing support for stem cells.
Bone marrow transplants are conducted for the treatment of severe bone marrow or blood cancer diseases. Often a cancer patient (leukemia or lymphoma) will remove their own bone marrow cells prior to a heavy dose of chemotherapy. The chemotherapy will kill off many cancer cells as well as healthy blood and immune cells. After chemo, the extracted bone marrow will be re-introduced back into the cancer patient’s bones, in order to repopulate the body with fresh blood and immune cells.
Bone marrow treatments are generally not regulated by the FDA. I am not entirely clear why bone marrow treatments are not considered a drug by the FDA, however, other autologous stem cell treatments mentioned below are currently being considered a drug. If someone has insight on this, please let me know.
Cord blood
Cord blood is found in the blood that remains in the placenta and umbilical cord after childbirth. Within this cord blood is a population of cord blood stem cells. It’s important to note that cord blood stem cells are a restricted stem cell population, similar to the hematopoetic stem cells in bone marrow. These cells can differentiate into blood and immune cells and a few other cell types.
Research is in progress to explore the use of cord blood to treat diabetes and certain blood cancers. Theoretically, cord blood stem cells can be used in the treatment of nearly 80 diseases, however it is unclear how effective cord blood is with these treatments. Blood clinics around the country now routinely treat patients with cord blood for hematological issues (anemia, leukemia, lymphoma). Cord blood functions similar to bone marrow cells, yet cord blood seems to be more immune tolerant (i.e. lower chance of chronic graft-versus-host disease).
When parents give birth to a child, they may be asked whether they’d like to extract cord blood and store the cells in a private cell bank. This is a tough decision. Storing cord blood is not cheap, but these cells may be useful some day for your child (autologous use) or for a related family member (allogenic use). Parents should know – there is currently a very low chance these stored cells will ever be utilized. The American Academy of Pediatrics notes that the odds of using a person’s own cord blood is 1 in 200,000. As stem cell therapy research continues into the future, it’s possible that these cells could be utilized to address a wide range of diseases (Parkinson’s, autism, Alzheimer’s, etc.) But for now, there are no approved stem cell treatments for these issues.
Despite a lack of approved therapies, a recent Phase 2 clinical trial from Duke University highlights the benefit of storing cord blood. Joanne Kurtzberg and her clinical team treated 63 children diagnosed with spastic cerebral palsy. Joanne’s team delivered between 10-50 million stem cells per kilogram of body weight via intravenous infusion. These children were treated with stem cells from their own umbilical cord blood. After a year, it was determined that children who received 25 million stem cells/kg. body weight showed the biggest improvements in motor function.
We are encouraged by the results of this study, which shows that appropriately dosed infusions of cord blood cells can help lessen symptoms in children with cerebral palsy
Joanne Kurtzberg (director of Duke’s Pediatric Blood and Marrow Transplant Program)
The FDA has approved multiple cord blood products.
As of writing (2020), there are currently 8 different cord blood approvals listed on the FDA page for approved cell therapy products (Allocord, Hemacord, Clevecord, Ducord, HPC, etc.) These treatments are described as ‘allogenic hematopoetic cell transplantations appropriate for patients suffering from a blood or immune cell disorder’. Allogenic means the collected cord blood stem cells can be used to treat people other than the donor (i.e. the parents could be treated with their child’s cord blood). In that sense, cord blood therapies could be thought of as a non-invasive version of a bone marrow transplant.
In 2011, Hemacord became the first FDA-approved stem cell product. Manufactured by the New York Blood Center, Hemacord is used for specified disorders in patients with problems in their blood-forming systems. In 2013, the name Hemacord changed to HPC Cord Blood.
Another cord blood product, HPC, is offered through Bloodworks in Seattle. Their website states they “collect 2 cups of cord blood” from the umbilical cord and placenta after pregnancy. Their cord blood product is used to treat various blood diseases.
Umbilical cord tissue
Human umbilical cord tissue is similar to cord blood, however the cord tissue is derived from the meaty center of an umbilical cord. Cord tissue seems to be a very rich source of mesenchymal stem cells. For this reason, these cells are often referred to as umbilical cord-derived mesenchymal stem cells (UC-MSCs). These particular stem cells appears to feature high cell vitality, low immunogenicity and high cell signalling (paracrine) potential to assist in various forms of tissue repair. This paper demonstrates how UC-MSCs significantly improved survival in mice suffering from acute lung injury. Human clinical trials are underway to determine whether the intravenous injection of UC-MSCs can offer symptom relief to Multiple Sclerosis patients. A group, led by Neil Riordan, already published their Phase 1/2 results. These preliminary results do look promising, but it’s important to recognize that this Multiple Sclerosis clinical trial was single arm, open label, single center and performed on only 20 MS patients. I realize this is clinical trial jargon, let me decipher. There was no placebo control group, both the patient and the physician knew the treatment they were delivering and this has only been tested on a small group of patients within a stem cell clinic in Panama. None of that discredits the results, but it means we need to wait for more rigorous clinical trials to know for certain whether stem cells offer effective treatment for MS patients. You can watch Neil Riordan and Mel Gibson discussing UC-MSC treatments in the adjacent video.
2020 update:
Multiple clinical trials have found mesenchymal stem cell (MSC) derived from umbilical cord (and other locations) are effective in treating neural damage in patients with multiple sclerosis.
Umbilical cord–derived MSCs are attractive treatment options for MS patients because they are extracted from easily attainable tissue, absent of any ethical dilemmas.
Upon intravenous injection, MSCs are able to traffic into the brain lesions and improve the survival rate of brain cells. Results also revealed that injection of MSCs decreases MS disease severity and improves quality of life in patients with MS. (reference)
This is incredibly encouraging. Currently, there are about 23 treatments for MS on the market but none of them are great. It’s interesting that these stem cells can travel to the area of the brain with MS lesions. If anyone knows how these MSCs target the lesions specifically, please let me know.
There are currently no FDA approved treatments that use human umbilical cord tissue.
This incredibly entertaining video features Joe Rogan and Mel Gibson (yes, that Mel Gibson) talking to Dr. Neil Riordan about UC-MSC treatments performed on Mel’s dad in Panama.
Amniotic stem cells
In early 2007, Dr. Anthony Atala and colleagues isolated a new type of stem cell from amniotic fluid. During pregnancy, amniotic fluid can be extracted from the amniotic sac without harming human embryos. Some scientists claim that stem cells found within amniotic fluid have more potential than the stem cells found in cord blood or bone marrow. More potential means these cells can differentiate into a wider variety of cells. It appears amniotic stem cells can develop into cells from all three developmental germ layers (endoderm, mesoderm, ectoderm). Importantly, they do not appear to form teratomas (a cancerous tumor formed from all 3 germ tissues).
These features make the amniotic stem cell population attractive for multiple medical applications, such as organ regeneration.
The adjacent TED Talk video features Dr. Anthony Atala speaking about organ regeneration. Amniotic stem cells are one of the sources of stem cells currently being used to build human tissue and human organs.
In 2009, the first U.S. amniotic stem cell bank opened in Medford, Massachusetts. This cell bank cryo-preserves and stores amniotic fluid stem cells.
Unfortunately, some stem cell clinics are putting the cart before the horse with these therapies. Two stem cell clinics in California have received an official complaint from the FTC (Federal Trade Commission) for using false and deceptive advertising to describe their stem cell therapies. The clinics treated patients with amniotic stem cells derived from the amniotic fluid of women who had cesarean sections. The clinics make outrageous and unfounded health claims (i.e. cure blindness, heal all organs) associated with the stem cell therapy.
There are currently no FDA approved amniotic stem cell therapy products.
Adipose stem cells (ASCs)
Adipose stem cells are a stem cell population that can be isolated from our fat. As strange as it sounds, the fat on our body (thighs, belly, ext.) is a rich source of stem cells. These ASCs are a form of mesenchymal stem cells. Bone marrow, cord blood and amniotic stem cells are also sources of mesenchymal stem cells. Similar to other mesenchymal stem cells, these ASCs are multipotent and can differentiate into many human cells (bone cells, cartilage cells, endothelial cells, adipocytes, muscle cells, ext.) Research is ongoing, but it appears these ASCs deliver an immunospuppresive effect, likely via the secretion of various trophic factors. Certain fractions of ASCs may also contain Tregs, a T cell that can suppress our immune system. For these reason, ASCs are being considered for autoimmune diseases or inflammatory disorders.
This is why there is so much interest in adipose stem cell treatment. In theory, if the science works…
- a physician could perform a liposuction on a patient
- the fatty tissue could be processed so as to isolate the adipose stem cells
- these ASCs could then be delivered to a part of the same patient’s body that is suffering from an inflammatory issue
One advantage to adipose stem cell treatment is the ASCs could be repeatedly harvested from fat in a relatively, non-invasive manner. ASCs represent a supply of mesenchymal stem cells that could be isolated from fat tissue in an easy manner. If you’re after mesenchymal stem cells, a liposuction is logistically easier than bone marrow, cord blood or amniotic fluid extraction.
Many organizations are currently pursuing clinical trials with ASCs. These trials span a broad range of applications, such as soft tissue regeneration, skeletal tissue repair, myocardial infarction, anti-inflammatory issues and pain management.
There are currently no adipose stem cell treatment products approved by the FDA.
South Korea, however, did approve an autologous, adipose-derived stem cell treatment in 2012. Anterogen, a Korea-based company, produced a stem cell product called Cupistem. Cupistem is based on the mesenchymal stem cells found in adipose tissue. Cupistem is approved by South Korea’s version of FDA for the treatment of Crohn’s fistula and for damaged or inflamed joint tissue.
2020 update: It should be noted that doctors continue to be skeptical whether adipose stem cells can be effectively used for regenerative medicine.
Dr. Mikail Kolonin with the McGovern Medical School at UTHealth studies fat tissue. “Very few clinical trials have demonstrated that those cells can do something useful. It’s not to say they can’t, but few clinical trials have been efficacy trials,” Dr. Kolonin said. He said there are no FDA-approved therapies using adipose stem cells now.
The rise of semi-legal, stem cell clinics in the United States
Stem cell science is plowing forward. As I highlight above, we are seeing some incredibly promising clinical trials. But, as of 2020, aside from cord blood and bone marrow, we have not seen any approved stem cell therapies on the market in the U.S.
Oddly enough, that market void is currently being filled with quasi-legal, stem cell clinics performing non-FDA approved procedures with questionable therapies.
In the past decade, if you desired a stem cell therapy, you would need to engage in stem cell tourism. Stem cell tourism entails international travel to China, Mexico, India, Panama, etc. in search of a clinician offering up a stem cell treatment. These days, this international effort is no longer necessary. A recent search identified 570 clinics in the United States currently selling stem cell therapies directly to consumers.
What are these stem cell clinics selling?
Most of these clinics are selling autologous treatments. As mentioned earlier, an autologous treatment involves removing stem cells from one part of your body, processing these cells in some way, then transplanting them back into the same individual. Many clinics are extracting fat and isolating adipose stem cells from the fat tissue. Our fat tissue (or adipose) are a rich source of stem cells. The technical term for the stem cells in our fat is adipose stem cells. According to this 2016 report, 61% of treatments offered by U.S. stem cell clinics are adipose stem cell treatments. Other commonly performed treatments utilize the hematopoetic stem cells found in bone marrow. Alright, so the clinics are mostly using stem cells from fat or bone marrow…
What physical ailments are treated by these clinics?
The main service offered by these clinics is relief from an orthopedic ailment. This orthopedic therapy usually involves using stem cells to reduce joint or soft tissue issues. The clinics claim their orthopedic stem cell treatments can reduce pain and inflammation. These claims may be true or they may not be true. The science is still inconclusive. Less scrupulous clinics, promise anti-aging, penis enlargement or treatment for Parkinson’s, Multiple Sclerosis, Cerebral Palsy and spinal cord damage. This is what happens in an unregulated environment, nothing is off the table.
The truth is …certain stem cell injections may be appropriate for a broad range of ailments, but professional scientists are still at work determining the safety and efficacy of the procedures.
We are still not sure what cell types will develop from various stem cells once they’re transplanted into certain parts of the body. These transplanted stem cells could form a cancerous tumor…or a serious immune-rejection issue…
A prudent consumer would wait till the kinks are ironed out.
Why isn’t the FDA cracking down on these non-approved stem cell clinics?
This is the real question. It isn’t surprising that a scrappy and agile business community is moving hard and fast to rake in money in the name of stem cells. That’s what hustlers do…they hustle. Some customers are paying 15K, out of pocket, for stem cell procedures. When there is demand, there will be supply.
However, it is surprising that the FDA is passively allowing the niche to develop.
In September 2019, the FDA published an updated warning document for people considering a non-approved stem cell therapy. In summary, they encourage consumers to ask clinics if the FDA has reviewed their treatments. The next question should focus on clinical trials. Has the clinic received an IND and is the stem cell therapy currently being tested in a clinical trial? If so, are there any results to review from these trials? If so, you can at least look for any safety issues. Personally, even if I was desperate, I would never undergo a treatment that had not first been explored in a clinical trial. Losing money on an experimental therapy is one thing, but subjecting yourself to dangerous side effects is a whole new level of risk.
So, what exactly is illegal about these non-approved stem cell treatments?
As of 2020, here is the latest FDA Industry Guidance on stem cell clinics. The FDA is focused on two criteria: minimal manipulation and homologous use.
In essence, the FDA will allow biological tissue (stem cells) to be applied only in the same part of the body from which the tissue was extracted. Also, the FDA will only allow minimal manipulation of a patient’s tissue. If a clinic is acting outside of these parameters, then they’re selling a drug and will require the appropriate licensing.
For example, it’s not legal for a clinic to remove fat tissue from a patient’s thigh, isolate out the adipose stem cells and then inject these stem cells into an elbow that is experiencing joint pain. That procedure would involve more than minimal processing and would shift the biological tissue from location A to location B. Therefore, that procedure is an infringement on the new FDA Industry Guidance.
What is the FDA doing to enforce illegal clinics?
I attended a medical conference in late 2019 and one of the keynote speakers was a high level FDA regulator. The speaker explained that the FDA simply doesn’t have enough budget and personnel to investigate and respond to the huge volume of semi-legal stem cell clinics. The FDA has a limited budget and already has a lot of drugs and food and supplements to monitor.
It should also be reiterated that the FDA is going after the most egregious offenders in the stem cell universe. For example, the FDA sent a stern warning letter to U.S. Stem Cell Clinic, an organization located in Sunrise, Florida. This particular clinic purportedly injected stem cells into the eyeballs of three elderly women. These women were seeking relief from macular degeneration. According to the New England Journal of Medicine, the stem cell treatment failed and led to blindness in these women.
The patients’ severe visual loss after the injection was associated with ocular hypertension, hemorrhagic retinopathy, vitreous hemorrhage, combined traction and rhegmatogenous retinal detachment, or lens dislocation.
Word to the wise, when dabbling in experimental and unproven treatments…. keep it out of your eyeballs.
In conclusion…
- Stem cell clinics are now located all across the U.S.
- Most of these clinics are operating outside of FDA regulations. They’re doing much more than minimal processing of biological tissue prior to transplanting. They’re also transplanting the processed stem cells into locations other then the area where the tissue was removed. Both of these actions render the treatment illegitimate by current FDA regulations.
- If you are considering a treatment at one of these clinics, ask to see their IND and clinical trial results.
I will continue to update this page as the field of stem cell science evolves. I hope this section shed some light on a question I hear often: Is stem cell therapy legal in the US?
To be continued…
References
Abbott, A. “Behind the scenes of the world’s first commercial stem-cell therapy.” Nature. doi 10 (2015).
Evans, Martin J., and Matthew H. Kaufman. “Establishment in culture of pluripotential cells from mouse embryos.” nature292.5819 (1981): 154.
Hirsch, Tobias, et al. “Regeneration of the entire human epidermis using transgenic stem cells.” Nature 551.7680 (2017): 327.
Hwang, Woo Suk, et al. “Patient-specific embryonic stem cells derived from human SCNT blastocysts.” Science 308.5729 (2005): 1777-1783.
Kuriyan, Ajay E., et al. “Vision loss after intravitreal injection of autologous “stem cells” for AMD.” New England Journal of Medicine 376.11 (2017): 1047-1053.
Makkar, Raj R., et al. “Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial.” The Lancet 379.9819 (2012): 895-904.
Schwartz, Steven D., et al. “Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies.” The Lancet385.9967 (2015): 509-516.
Sun, Jessica M., et al. “Effect of Autologous Cord Blood Infusion on FDA approved stem cell therapies in 2019 with Cerebral Palsy: A Randomized, Placebo‐Controlled Trial.” Stem cells translational medicine 6.12 (2017): 2071-2078.
Tachibana, Masahito, et al. “Human embryonic stem cells derived by somatic cell nuclear transfer.” Cell 153.6 (2013): 1228-1238.
Takahashi, Kazutoshi, et al. “Induction of pluripotent stem cells from adult human fibroblasts by defined factors.” cell 131.5 (2007): 861-872.
Turner, Leigh, and Paul Knoepfler. “Selling stem cells in the USA: assessing the direct-to-consumer industry. Is stem cell therapy legal in the US?” Cell Stem Cell 19.2 (2016): 154-157.
Tsuji, Wakako, J. Peter Rubin, and Kacey G. Marra. “Adipose-derived stem cells: Implications in tissue regeneration.” World journal of stem cells 6.3 (2014): 312.
Zhu, Hua, et al. “Therapeutic Effects of Human Umbilical Cord-Derived Mesenchymal Stem Cells in Acute Lung Injury Mice.” Scientific reports 7 (2017): 39889.